Home / Educational / Mandatory Biennial Count: Step-by-Step Guide
Per the Code of Federal Regulations Title 21 Chapter 2 section 1304.11, “after the initial inventory is taken, the registrant shall take a new inventory of all stocks of controlled substances on hand at least every two years.”
After the initial physical inventory recording, this biennial recording becomes the foundation on which each future count can be looked back for reference.
Although the law states biennial counts must happen ‘at least every 2 years’, it also states they can be done on a sooner timeframe. The regulation reads “the biennial inventory may be taken on any date which is within two years of the previous biennial inventory date.” This is important to note because while the longest amount of time “allowed” may be 2 years, you really do not want to wait 2 years to find there is a large discrepancy that could have been caught and handled much sooner had there been more frequent physical counts. Some other time frames to consider would be monthly, every 3 months, twice yearly, and yearly.
How should you perform a regular biennial count?
These include:
- The name of the substance
- Finished form of the substance (e.g., 10-milligram tablet or 10-milligram concentration per fluid ounce or milliliter);
- The number of units or volume of each finished form in each commercial container (e.g., 100-tablet bottle or 3-milliliter vial)
- The number of commercial containers of each such finished form (e.g. four 100-tablet bottles or six 3-milliliter vials).
Step 1: Prepare before the day of count (reconcile logbook if needed)
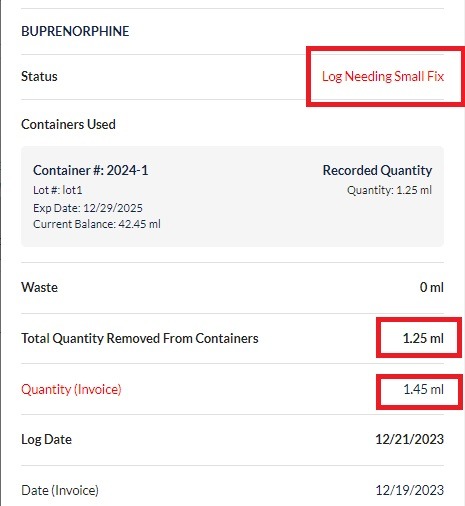
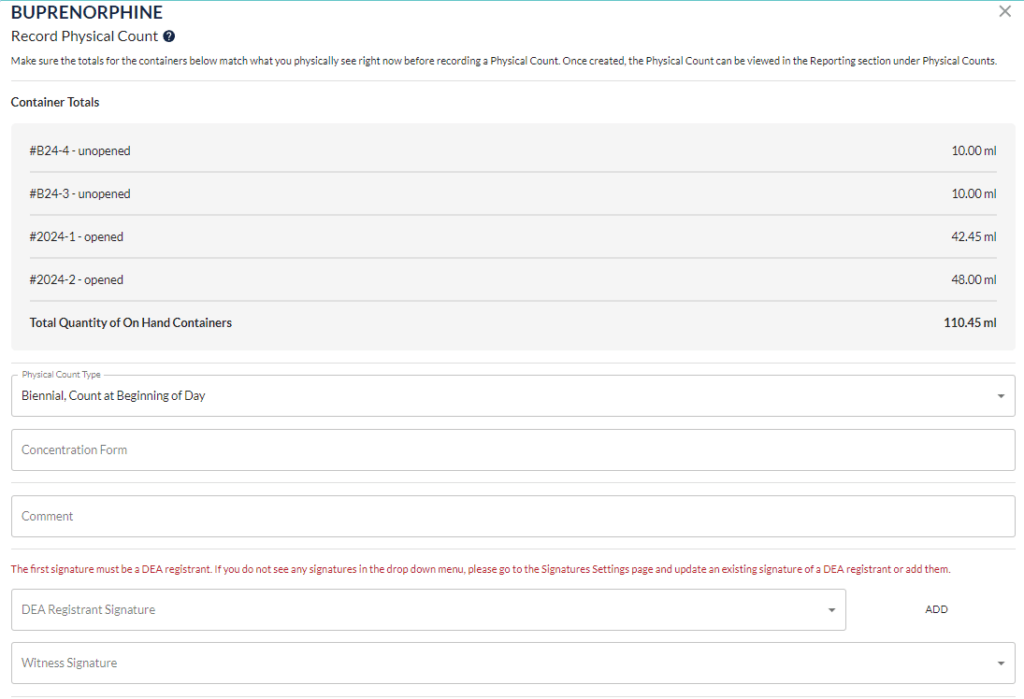
Step 2: Start with one drug at a time. The DEA registrant and a secondary witness need to count all unopened container quantities. Next count all opened container quantities. Add these two quantities together to get the total quantity on hand of this drug.
Step 3: Record this number and all other required details following the list of requirements provided above. Both parties involved in the count will need to sign the document.
Step 4: Repeat for all remaining controlled substances
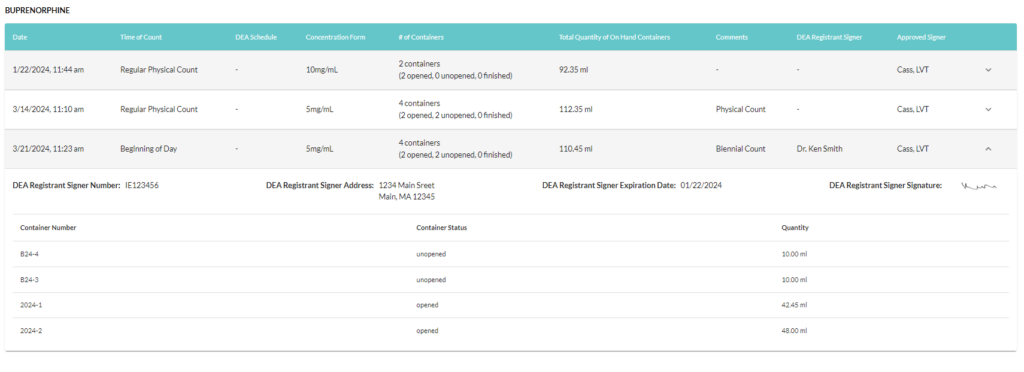
Step 5: Maintain this record of reference for 2 years per CFR Title 21 Chapter 2 section 1304.04
If you are using VetSnap for your controlled substances logging and management there is a way to simplify the biennial counts process!
The biennial counting process is no small task, and it’s no wonder hospitals try to avoid it. But it’s an important part of the compliance process which proves that you actually care about compliance, tracking your controlled substances, and are reliable DEA registrants.
