Home / Are You Following These Best Practices to Stay Compliant?
The DEA requirements are that the entire life span of each container that enters your hospital is thoroughly recorded. Are you implementing these important steps into your practice correctly?
Once a controlled drug container arrives at your hospital, you should be recording the information of that particular container:
Every container must also be tracked in regards to what occurs with them, in addition to log entries.
Any usage from a container must be detailed
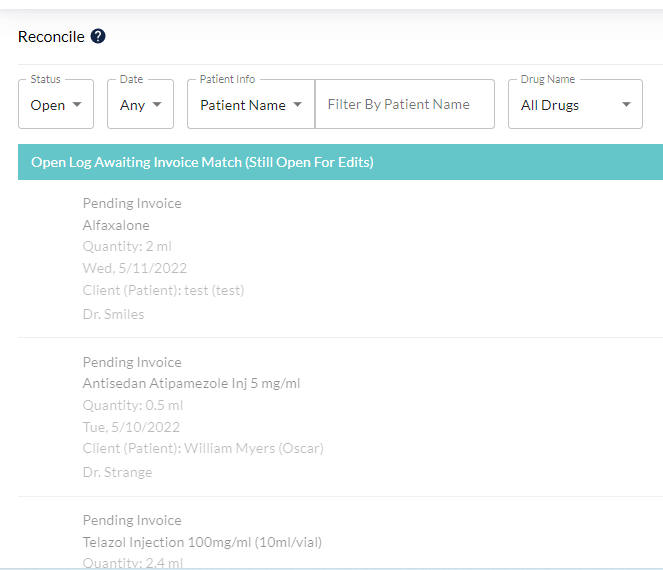
Reconciliation to verify correct amounts have been recorded, and balances shown are accurate
Recording waste
Finishing a container
VetSnap makes it easier to duplicate container information (invoice number, NDC/NADA number, lot number, expiration date) with the touch of a button

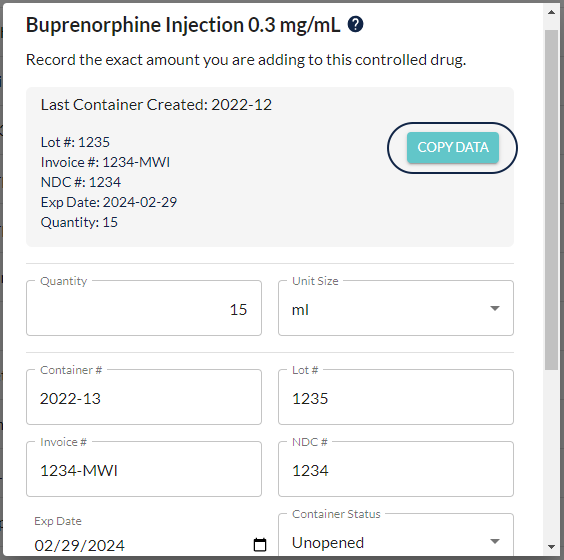

VetSnap makes it easier to duplicate container information (invoice number, NDC/NADA number, lot number, expiration date) with the touch of a button
Support for customers
Current VetSnap customers that need help can reach out to support@vetsnap.com with your ask, hospital name, hospital zip code, and preferred email contact.
Get Social with VetSnap
Terms of Use
(C) 2025 VetSnap Corporation. All rights reserved.
Controlled Substances DEA Compliance Digital Logbook LogButler