Home / Educational / What should you look for in a controlled log book solution for your veterinary hospital?
"While paper logs may still be prevalent in hospitals, having the right tool for compliance will bring a much higher likelihood for success and make the process easier for your team. Much like choosing the right tools for a procedure, or choosing the right medication for an illness, choosing the right logbook solution like VetSnap can mean the difference between a passing and failing audit"
The Essential Features You Should Look for in a Digital Controlled Logbook Solution:
Meticulous Container Inventory Tracking
At the heart of a robust controlled substance management strategy lies detailed container tracking. As soon as a controlled substance shipment arrives, each container must be meticulously logged into an Unopened Container Log. This practice enables an accurate and readily available inventory snapshot, providing an immediate resource for identifying potential discrepancies.
Opened Container Tracking
Once a container transitions from unopened to opened, each dosage use should be vigilantly tracked until the container reaches depletion. This Opened Container Log protects against unauthorized access and misuse, promotes accurate dosage administration, and serves as an invaluable asset for maintaining a precise record of controlled substances.
Thorough Waste Tracking
A well-structured logbook solution must prioritize the tracking of waste, capturing essential details like the container, date, reasons for wastage, and the responsible parties. Proper waste tracking demands double confirmation of signatures to witness the disposal of controlled drugs. It should provide a detailed and easily accessible audit trail when examining your logbook.
Activity Tracking with Signatures
Establishing a clear signature approval process, especially when controlled drugs are involved, is crucial. A system requiring a verified practitioner’s signature for each use of a controlled substance creates a traceable chain of custody, instilling a strong sense of accountability within your team. Make sure it can track any changes made to your log entries. This process also produces an invaluable history trail, vital during audits or investigations.
Regular Reconciliation
Even amidst busy schedules, regular reconciliation is an essential yet often overlooked logging process. This method involves a thorough comparison of your controlled substances logs against your Practice Information Management System (PIMS) records and your on-hand balances in all containers, including unopened, opened, and finished bottles. Regular and proper reconciliations, approved by the appropriate hospital personnel, ensure up-to-date records and keep discrepancies to a minimum.
VetSnap: Your Comprehensive Digital Controlled Logbook Solution
VetSnap’s leading controlled drug logging solution excels at helping a hospital stay compliance and implement an effective record keeping process that address all the best practices above.
Streamlined Container Tracking
With VetSnap, container tracking becomes an effortless task. The software’s digital logs offer real-time tracking of both unopened and opened containers. Automatic reconciliation with PIMS minimizes the risk of human error, providing a reliable snapshot of your controlled substances.
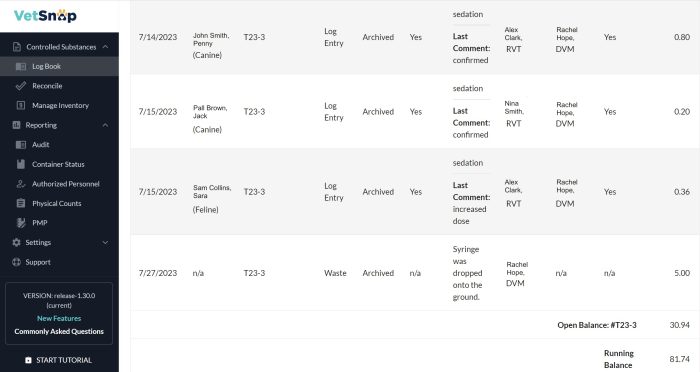
Seamless yet Simple Discrepancy Tracking, e.g., Waste
VetSnap provides a clear paper trail of accountability when hospital staff need to record and explain for the unpredictable, such as accidental breakages or drug expiration. Clarity and detail will help with any internal or external reviews or audits.
Efficient Log Creation
VetSnap facilitates the creation of compliant log entries, speeding up the process by up to 75% compared to manual methods. Its intuitive design ensures legible, complete, and error-free logs. By simplifying the logging process, VetSnap encourages your team to more effortlessly maintain an up-to-date logbook record.
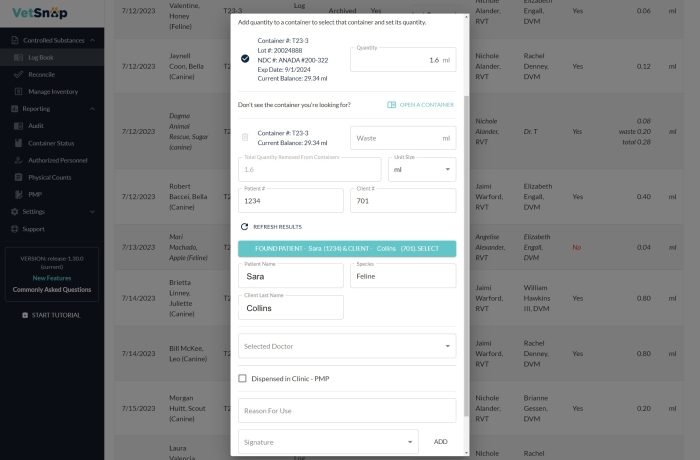
Promoting Accountability
VetSnap’s digital signature feature records and verifies every controlled substance use by an authorized practitioner. This promotes accountability and ensures that only authorized personnel administer these substances. Moreover, VetSnap’s permissions and roles can be tailored to align with your hospital’s internal trust hierarchy, from basic logging to reconciliation to waste tracking.
Seamless Regular Reconciliation
VetSnap significantly eases the reconciliation process by automatically cross-referencing controlled drug log entries with corresponding invoices and PIMS records. It further enhances accuracy by tracking each bottle through its lifecycle, ensuring proper documentation of any overages, underages, and discrepancies. Its day-by-day audit reporting feature, mimicking the review methods of DEA agents, helps hospital teams stay reconciled and audit-ready. By identifying discrepancies and suggesting resolution options, VetSnap aids in maintaining accurate and up-to-date logs, significantly reducing the risk of DEA fines.
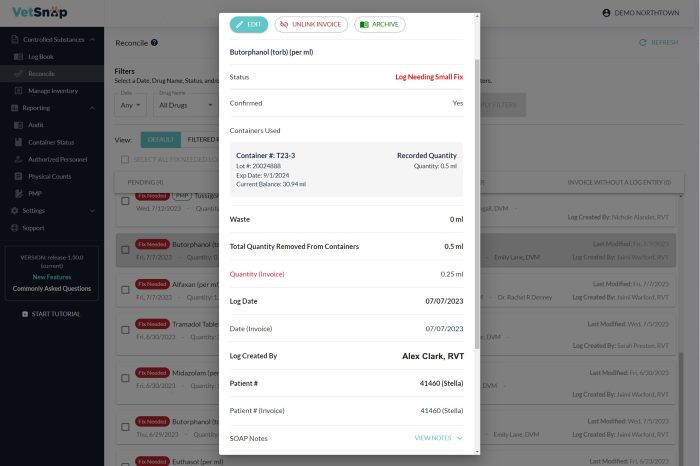
Audit-Ready DEA Compliance Reports
VetSnap streamlines audit procedures by offering accessible, comprehensive records. Features like container status history reports, log entry reports, and biennial reporting records can be printed at any time, easing the process of retrieving records for auditors. VetSnap’s advanced search capabilities enable quick responses to any follow-up queries or more detailed requests from auditors.
In summary, VetSnap delivers all the features integral to a reliable controlled logbook solution. Its extensive suite of features and automation capabilities align seamlessly with best practices and allows hospitals to stay compliant with more ease and less stress.


